Hafiz Khalid, Director of Product Marketing, XP Power
Given the continuous development of new technologies and new equipment in the field of medicine, there is a growing market for robust and reliable power solutions for a segment that perhaps represents the culmination of what is considered a “critical mission.” This article explains five design aspects that engineers should consider when designing specifically for medical applications.
Long gone are the days when a doctor's equipment consisted mainly of a stethoscope, a manual blood pressure meter and his two fingers to check the heart rate. Medical personnel are now more likely to use a cart with sophisticated electronic equipment that can monitor and measure various vital signs.
The market for this equipment has grown enormously in recent years as demand for healthcare has increased, both in clinical settings and at home. It was valued at about $1.500 billion in 2022 and is expected to experience average year-on-year growth of around 6,5% until 2027, according to the analyst firm Markets and Markets, mainly due to the aging population and emerging markets. For example, in 2023, around 10% of the world's population was over 65 years old but by 2100 this figure is expected to be 26%.
Medical equipment designs must address very different publications
The design of medical equipment is highly regulated and focused on safety. However, standards must cover a wide variety of equipment types and usage environments, from battery-powered monitors and multi-kW scanners to operating rooms, analysis laboratories, and point-of-care services. These diverse conditions are contemplated in the IEC 60601-1 standard and its versions for each country, making it a complex and extensive document.
At the same time, there is inevitable commercial pressure for equipment costs to be low and for products to include automation, better functionality and, today, AI. All this in order to improve patient outcomes and streamline workflows, especially in the analysis of biological samples and clinical data.
- Risk assessment
The most recent editions of IEC 60601-1 require risk assessment and management and constitute a starting point for the design of medical products. The manufacturer must decide whether the product could realistically come into contact with patients and apply appropriate levels of protection. The standard distinguishes between a “medical device” (MD) that may be in contact with a patient and an “in vitro diagnostic medical device” (IVD MD) such as a centrifuge or blood analyzer, which normally would not be. The risk management procedure must follow the structures of ISO 14791, “Application of risk management to MDs” and the requirements of the document cover the entire product life cycle. It contemplates very diverse aspects, including risks such as biocompatibility, data and system security, electricity, moving parts, radiation and suitability for use. Although not all of them may be applicable to power supplies, whether external or built-in, the increasing use of digital control and monitoring in power designs represents a new area in which the risk of accidental data corruption or its deliberate manipulation with software/firmware must be evaluated and mitigated.
Note that ISO 14971 only provides an environment and does not specify hazards, what risk is acceptable, or how to quantify the risk. The Figure 1 represents a typical risk management workflow.
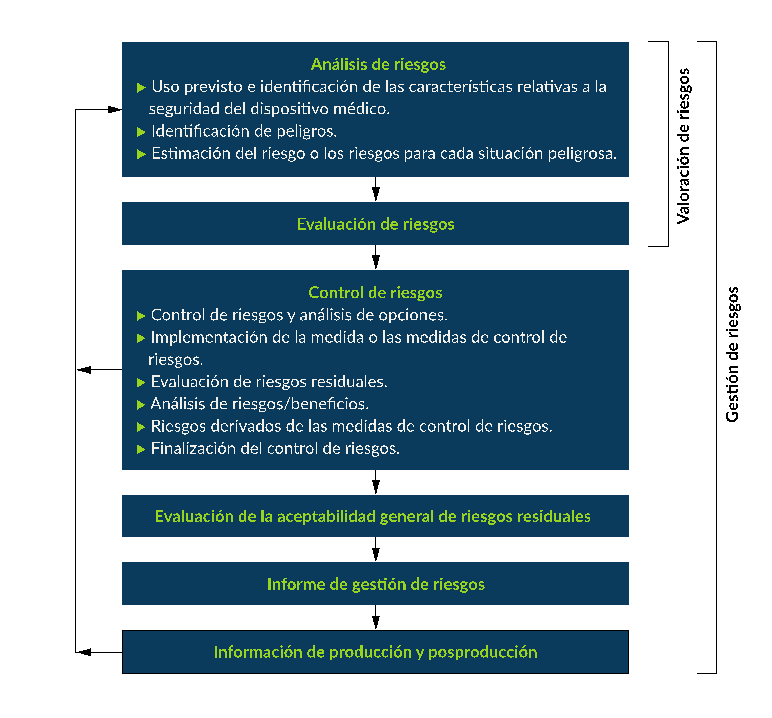
Figure 1: Typical risk management process according to ISO 14971.
- Safety is priority
Among all medical equipment requirements, safety is number one and in practice includes protection against electric shock, fire, and mechanical hazards, among others. The IEC 60601-1 medical safety standard has adopted the term “Means of Protection” (MOP), so that at least two MOPs are required to prevent a shock, following the principle that two failures should occur before the equipment could be unsafe. This can be compared to other safety standards that require reinforced insulation, equivalent to two separate levels of protection, or one reinforced performance level, such as thick, solid insulation.
The standard defines the operator and patient environments, as well as air clearances and insulation creepage distances, which are different for the two. For example, two Means of Patient Protection (2 x MOPP) require a creepage distance of 8mm and a test voltage of 4kVAC for system voltages up to 250VAC. When connection to the patient has been planned or is possible, the medical safety standard requires taking into account the possible failure of other equipment that could leave the patient “connected” to the mains voltage. In this case there should be no connection path through normally functioning equipment to the ground that allows the circulation of a lethal current. In practice, this means providing at least 1 x MOPP insulation between the patient connections and the ground on medical equipment. This requirement is usually not met by power supplies conforming to IEC 62368-1 (the standard for audio/video, information and communication technology equipment), where the output may consist of, at most, functional isolation up to the socket. of Earth.
The same principle applies to unspecified external signal connections to patient-connected medical equipment and its power sources. In this case, 2 x MOPP isolation is required from these lines to the connections to the patient since it must be assumed that the signal lines could be connected to the AC network through other faulty equipment. If connected equipment is specified, 1 x MOPP to interface is acceptable. Low power DC/DC converters with 1 x 2 x MOPP isolation and very low coupling capacity are sometimes added to the power lines to meet these requirements (Figure 2).
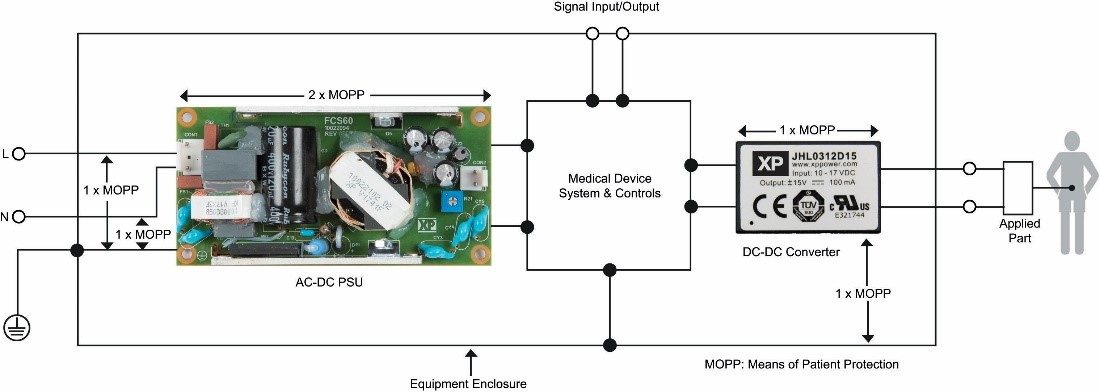
Figure 2: Configuration of a power unit and connected equipment set with their MOPP levels.
The insulation requirements for operator handling (Means of Operator Protection or MOOP) are like those of IEC 62368-1 but there are differences; for example, in fuse configurations and allowable leakage current.
- Leakage current is a key factor
Safety specifications generally refer to electrical insulation, but the laws of Physics dictate that there will always be a certain level of leakage current between the AC mains connections and the case or output of a power supply, regardless. through stray coupling capacitance or EMI suppression capacitors. In medical applications, the tolerable level of patient leakage current through the power supply is case-by-case. The specified levels are for normal and single fault conditions, and may be as low as 10µA, for example, when the equipment has direct connections to the heart, such as with the “cardiac floating” (CF) connection. The Table 1 shows the supported levels.
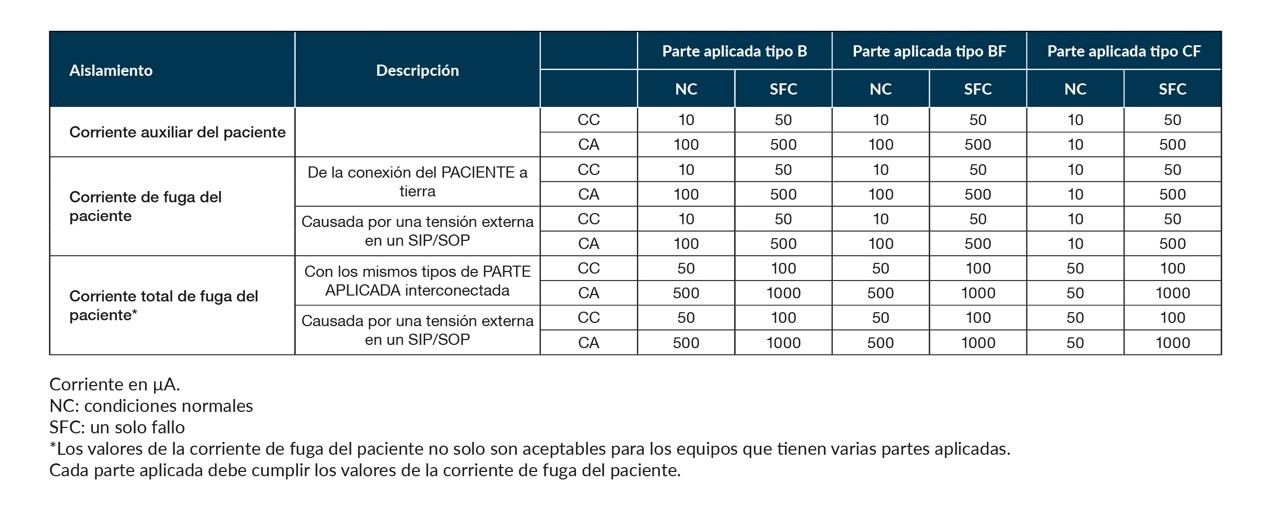
Table 1: Permissible “auxiliary” and leakage current levels according to IEC 60601-1.
Medical equipment that only requires MOOP, such as those used in laboratories, must also have a low total ground leakage current of 0,5mA under normal conditions (0,3mA in the US). Compare the limit for Power Source 2 (ES2) of up to 5mA at 50/60Hz according to IEC 62368-1.
- EMC limits must be met
IEC 60601-1-2 indicates the electromagnetic compatibility (EMC) requirements of medical devices based on CISPR11 and the IEC 61000-4-x series of EMC standards, which covers the same emissions and immunity tests for commercial, industrial equipment and about you. In edition 4.1 of IEC 60601-1-2, the most recent, a new test for immunity and proximity to magnetic fields in the frequency range of 9kHz to 13,56MHz has been added. This addresses potential sources of interference, such as those from wireless devices in various environments where medical equipment, home care and assisted living facilities, offices, public spaces, emergency vehicles and hospitals are used.
Another significant change in this edition was to require testing for emissions, AC droops and signal losses at the extremes of the AC voltage range, and not just for the nominal voltage, again reflecting that the operating environment can be very varied. The IEC 61000-4-x series includes various severity/immunity levels and IEC 60601-1-2 details which one to use, depending on the application and intended medical environment.
It is well known that leakage current and low conducted EMI tend to be mutually exclusive because common-mode EMI-attenuating “Y” capacitors also couple leakage current into a grounded case or outputs. This has oriented the design of power converters towards topologies that are inherently less noisy, such as resonant types. Fortunately, this usually leads to greater efficiency and its corresponding advantages.
- Functionality must be taken into account
Since medical equipment is found in all healthcare facilities, it must be practical; that is, light, silent, easy to feed or recharged and robust. They must resist knocks and falls due to continuous use and must resist the action of fluids and other materials that are usually present in healthcare environments.
What increases the effectiveness of modern equipment is its ability to collect and analyze data, perhaps with wireless connections to aggregate information or allow remote monitoring or diagnosis. In homes it could be done through a Bluetooth connectionTM to an app and, in the laboratory or diagnostic center, a network connection to local servers or the cloud is more common. In this setup, workflow speed and efficiency are key performance indicators and monitoring equipment effectiveness is vital to ensure maximum uptime and confidence in the data collected. Equipment power supplies that provide digital monitoring of their outputs, with advance warning of an AC failure and performance degradation, are invaluable in this situation. In addition, the remote precision control of parameters such as voltage or output current facilitates the automation of equipment such as the control of the laser diode current in microsurgery or aesthetic treatments.
An example of a power supply suitable for medicine
A power supply well suited for medical and industrial applications is the HPKF3K0 series from XP Power (Figure 3). Suitable for high power applications with its 3kW power rating, this unit features 2 x MOPP insulation with a leakage current of less than 500µA, making it suitable for many applications. It meets EMC specifications for medicine and incorporates sophisticated digital control and monitoring functions such as programmable voltage 0-105% and programmable current 0-110% through its digital interface, which is compatible with PMBus, CANopen, MODBUS and SCPI protocols. to offer maximum flexibility. The HPF3K0 series has medical certifications for global markets and is supplied with an input range of 90-264VAC and fixed outputs of 24V, 36V, 48V or 60VDC nominal. Its efficiency is up to 93% and its size is 279,4 x 177,8 x 63,5mm (11 x 7 x 2,5 inches).

Figure 3: XP Power's HPF3K0 3kW power supply is intended for medical applications.
Conclusions
We have discussed five aspects that must be taken into account when designing a medical product, and specifically its power supply. There are many more and the specifications to meet can be overwhelming, but the goal is to ensure that product designs are safe in all environments and that the patient experience is better. Power supplies certified to medical standards from global manufacturers provide a secure foundation for new designs.